27++ Gibbs free energy animation ideas in 2021
Home » Wallpapers » 27++ Gibbs free energy animation ideas in 2021Your Gibbs free energy animation images are ready in this website. Gibbs free energy animation are a topic that is being searched for and liked by netizens today. You can Find and Download the Gibbs free energy animation files here. Get all royalty-free photos and vectors.
If you’re looking for gibbs free energy animation pictures information related to the gibbs free energy animation topic, you have visit the right site. Our website always provides you with suggestions for viewing the maximum quality video and picture content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
Gibbs Free Energy Animation. See page 566 04 Question 2 points Watch the Chem Tour animation below on Gibbs Free Energy before answering the following questions. 4 Monifaasisysteemien laskenta rajoitettua Gibbsin energian minimointia käyttäen Introduction to constrained Gibbs energy methods in process and materials research. The thermodynamic calculations are done with a Gibbs free energy minimization model 13 using the predictive Soave-Redlich-Kwong Equation of state to calculate the required fugacity coefficients. Kersten in Handbook of Biofuels Production 2011 203 Chemical thermodynamics.
 Second Law Of Thermodynamics Entropy Gibbs Free Energy Youtube From youtube.com
Second Law Of Thermodynamics Entropy Gibbs Free Energy Youtube From youtube.com
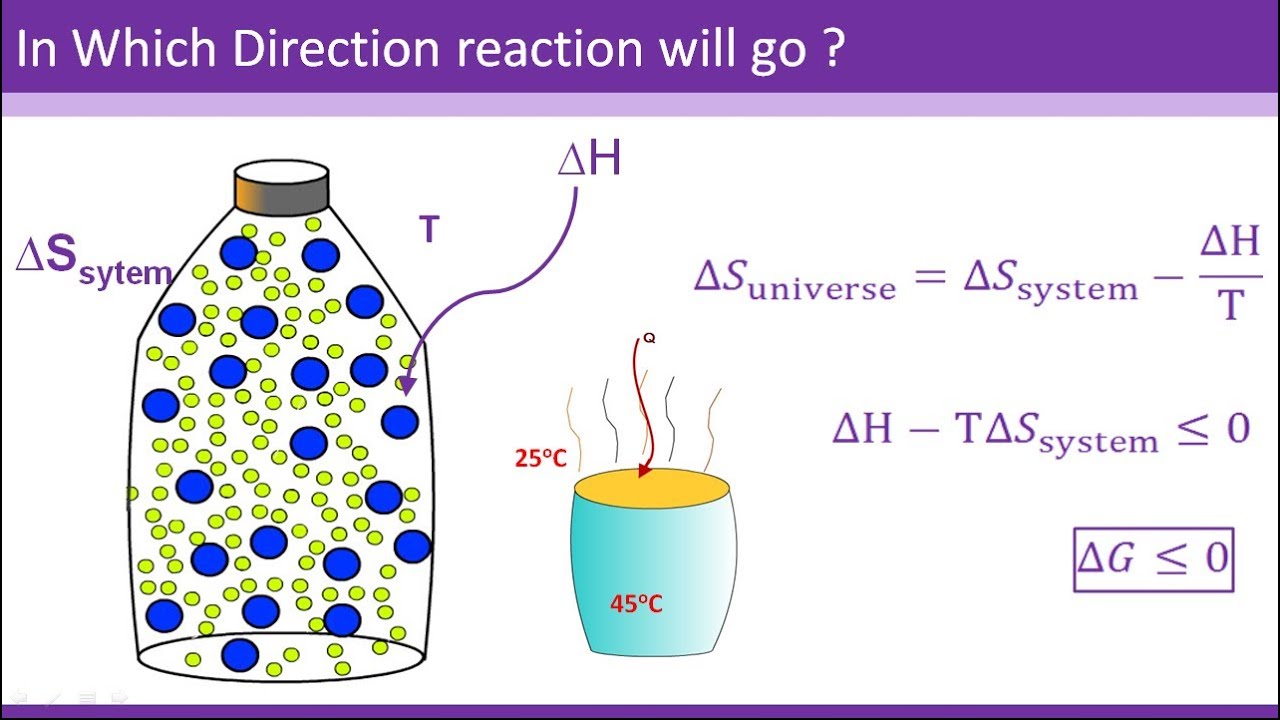
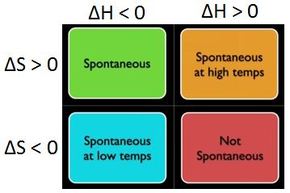
Paul Andersen attempts to explain Gibbs Free Energy. Gibbs free energy reaches a minimum point in composition space and then it will become stuck. Gibbs Free Energy. The change in free energy ΔG is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system. Create animated videos a. Explanation of spontaneous process.
Coke deposition the content of methane and carbon dioxide in syn-gas as well as H 2 CO ratio were investigated as a function of CO 2 CH 4 and H 2 OCH 4 mole ratios at different temperatures and pressures.
This functionality can be formally written as. In thermodynamics the Gibbs free energy or Gibbs energy is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressureThe Gibbs free energy measured in joules in SI is the maximum amount of non-expansion work that can be extracted from a thermodynamically closed system one. This functionality can be formally written as. Condition for spontaneity and Gibbs free energy for class XI students CBSE. The CPU times were 547 s and 525 s for parameters a and b respectively. 14 In the thermodynamic calculations the non-gasified part of the feedstock remains as.
 Source: giphy.com
Source: giphy.com
He begins by using three spontaneous reactions to explain how a change in enthalpy entropy and temperature can affect the free energy of a system. From h f o bio and s f o b i o it is possible to calculate the standard molar Gibbs free energy of formation of microorganisms from elements g f o b i o using the Gibbs equation 14 g f o b i o h f o b i o T s f o b. The thermodynamic calculations are done with a Gibbs free energy minimization model 13 using the predictive Soave-Redlich-Kwong Equation of state to calculate the required fugacity coefficients. A brief and simple way of explaining Gibbs Free Energy– Created using PowToon. Explanation of spontaneous process.
 Source: in.pinterest.com
Source: in.pinterest.com
This functionality can be formally written as. The Gibbs free energy is a function of pressure temperature and composition ie the moles of the various components that are present eg H2O CO2 etc. Consider the change in Gibbs free energy when we mix two components to form a regular solution. To get an overview of Gibbs energy and its general uses in chemistry. This functionality can be formally written as.
 Source: surfguppy.com
Source: surfguppy.com
The reason is that one involves the volume. He begins by using three spontaneous reactions to explain how a change in enthalpy entropy and temperature can affect the free energy of a system. Paul Andersen attempts to explain Gibbs Free Energy. Oftentimes in biochemistry we write energy requiring processes such as in the conversion of a monomer to a larger polymer as one-step reactions that are ultimately fueled by separate reaction involving the breakdown or hydrolysis remember hydrolysis just means reaction with water of ATP to produce adp and a free phosphate group now when we write it this way it implies that the breakdown of ATP. Gibbs free energy denoted G combines enthalpy and entropy into a single value.
 Source: slidetodoc.com
Source: slidetodoc.com
Create animated videos a. The Gibbs free energy is a function of pressure temperature and composition ie the moles of the various components that are present eg H2O CO2 etc. Explanation of spontaneous process. Gibbs free energy denoted G combines enthalpy and entropy into a single value. Gibbs free energy.
 Source: youtube.com
Source: youtube.com
Indias best GATE Courses with a wide coverage of all topicsVisit now and crack any technical exams httpswwwgateacademyshopFor various online courses b. GIBBS FREE ENERGY The Second Law Of Thermodynamics M PI Test your understanding of the second law by answering the following question. From h f o bio and s f o b i o it is possible to calculate the standard molar Gibbs free energy of formation of microorganisms from elements g f o b i o using the Gibbs equation 14 g f o b i o h f o b i o T s f o b. A brief and simple way of explaining Gibbs Free Energy– Created using PowToon. Willard Gibbs 18381903 an American physicist who first developed the concept.
 Source: youtube.com
Source: youtube.com
Keywords Gibbs free energy constrained minimization immaterial constraint work-coefficient extent of reaction paraequilibrium. Condition for spontaneity and Gibbs free energy for class XI students CBSE. Dependence of Gibbs Energy on Temperature and Pressure. The Gibbs free energy G often called simply free energy was named in honor of J. Gibbs free energy reaches a minimum point in composition space and then it will become stuck.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The reason is that one involves the volume. To get an overview of Gibbs energy and its general uses in chemistry. Gibbs free energy minimization was applied to study thermodynamic equilibrium of the combined steam and carbon dioxide reforming of methane. Condition for spontaneity and Gibbs free energy for class XI students CBSE. Consider the change in Gibbs free energy when we mix two components to form a regular solution.
 Source: youtube.com
Source: youtube.com
To get an overview of Gibbs energy and its general uses in chemistry. Condition for spontaneity and Gibbs free energy for class XI students CBSE. Gibbs Energy - Gibbs free energy is a very useful property it decreases for a spontaneous process at constant temperature and pressure. The Gibbs free energy is a function of pressure temperature and composition ie the moles of the various components that are present eg H2O CO2 etc. Knowledge of free energy under one condition is compared with another allows us to predict the direction of.
 Source: shefalitayal.com
Source: shefalitayal.com
The Gibbs free energy G often called simply free energy was named in honor of J. Kersten in Handbook of Biofuels Production 2011 203 Chemical thermodynamics. Willard Gibbs 18381903 an American physicist who first developed the concept. A brief and simple way of explaining Gibbs Free Energy– Created using PowToon. GIBBS FREE ENERGY The Second Law Of Thermodynamics M PI Test your understanding of the second law by answering the following question.
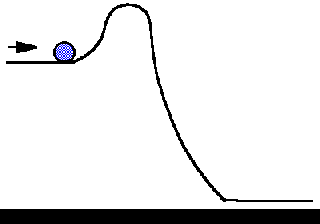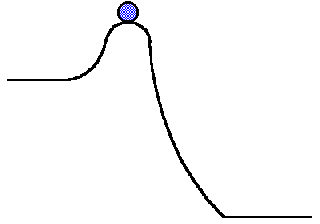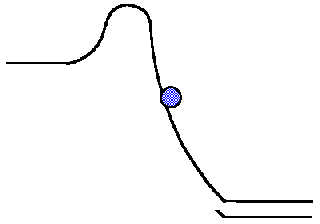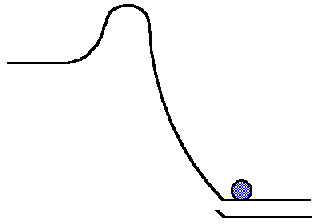 Source: employees.csbsju.edu
Source: employees.csbsju.edu
Explanation of spontaneous process. From h f o bio and s f o b i o it is possible to calculate the standard molar Gibbs free energy of formation of microorganisms from elements g f o b i o using the Gibbs equation 14 g f o b i o h f o b i o T s f o b. Coke deposition the content of methane and carbon dioxide in syn-gas as well as H 2 CO ratio were investigated as a function of CO 2 CH 4 and H 2 OCH 4 mole ratios at different temperatures and pressures. 14 In the thermodynamic calculations the non-gasified part of the feedstock remains as. Consider the change in Gibbs free energy when we mix two components to form a regular solution.
 Source: shefalitayal.com
Source: shefalitayal.com
The Gibbs free energy is a function of pressure temperature and composition ie the moles of the various components that are present eg H2O CO2 etc. 4 Monifaasisysteemien laskenta rajoitettua Gibbsin energian minimointia käyttäen Introduction to constrained Gibbs energy methods in process and materials research. The change in free energy ΔG is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system. The thermodynamic calculations are done with a Gibbs free energy minimization model 13 using the predictive Soave-Redlich-Kwong Equation of state to calculate the required fugacity coefficients. Create animated videos a.
 Source: slideplayer.com
Source: slideplayer.com
To get an overview of Gibbs energy and its general uses in chemistry. Willard Gibbs 18381903 an American physicist who first developed the concept. A brief and simple way of explaining Gibbs Free Energy– Created using PowToon. The thermodynamic calculations are done with a Gibbs free energy minimization model 13 using the predictive Soave-Redlich-Kwong Equation of state to calculate the required fugacity coefficients. The Gibbs free energy is a function of pressure temperature and composition ie the moles of the various components that are present eg H2O CO2 etc.
 Source: youtube.com
Source: youtube.com
Gibbs free energy minimization was applied to study thermodynamic equilibrium of the combined steam and carbon dioxide reforming of methane. Enthalpy temperature and entropy. See page 566 04 Question 2 points Watch the Chem Tour animation below on Gibbs Free Energy before answering the following questions. The Gibbs free energy G often called simply free energy was named in honor of J. Paul Andersen attempts to explain Gibbs Free Energy.
 Source: youtube.com
Source: youtube.com
The Gibbs free energy is a function of pressure temperature and composition ie the moles of the various components that are present eg H2O CO2 etc. From h f o bio and s f o b i o it is possible to calculate the standard molar Gibbs free energy of formation of microorganisms from elements g f o b i o using the Gibbs equation 14 g f o b i o h f o b i o T s f o b. Condition for spontaneity and Gibbs free energy for class XI students CBSE. The reason is that one involves the volume. He then applies this concept to.
 Source: crediblehulk.org
Source: crediblehulk.org
Willard Gibbs 18381903 an American physicist who first developed the concept. See page 566 04 Question 2 points Watch the Chem Tour animation below on Gibbs Free Energy before answering the following questions. Indias best GATE Courses with a wide coverage of all topicsVisit now and crack any technical exams httpswwwgateacademyshopFor various online courses b. Willard Gibbs 18381903 an American physicist who first developed the concept. The thermodynamic calculations are done with a Gibbs free energy minimization model 13 using the predictive Soave-Redlich-Kwong Equation of state to calculate the required fugacity coefficients.
 Source: study.com
Source: study.com
The reason is that one involves the volume. From h f o bio and s f o b i o it is possible to calculate the standard molar Gibbs free energy of formation of microorganisms from elements g f o b i o using the Gibbs equation 14 g f o b i o h f o b i o T s f o b. Gibbs Energy - Gibbs free energy is a very useful property it decreases for a spontaneous process at constant temperature and pressure. Knowledge of free energy under one condition is compared with another allows us to predict the direction of. The change in free energy ΔG is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system.
 Source: teksguide.org
Source: teksguide.org
Knowledge of free energy under one condition is compared with another allows us to predict the direction of. He then applies this concept to. The CPU times were 547 s and 525 s for parameters a and b respectively. See page 566 04 Question 2 points Watch the Chem Tour animation below on Gibbs Free Energy before answering the following questions. GIBBS FREE ENERGY The Second Law Of Thermodynamics M PI Test your understanding of the second law by answering the following question.
 Source: medium.com
Source: medium.com
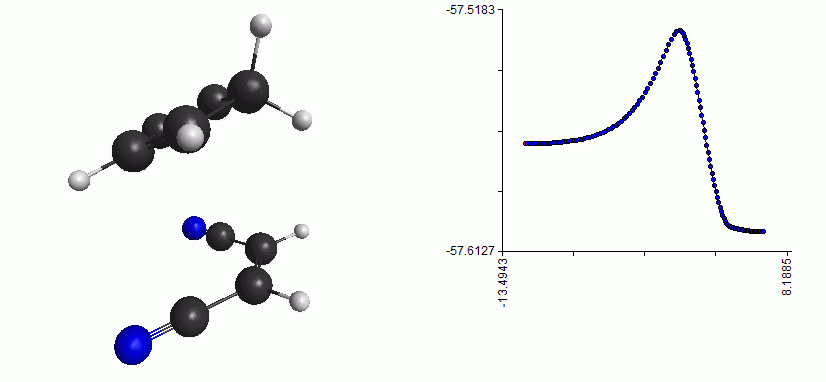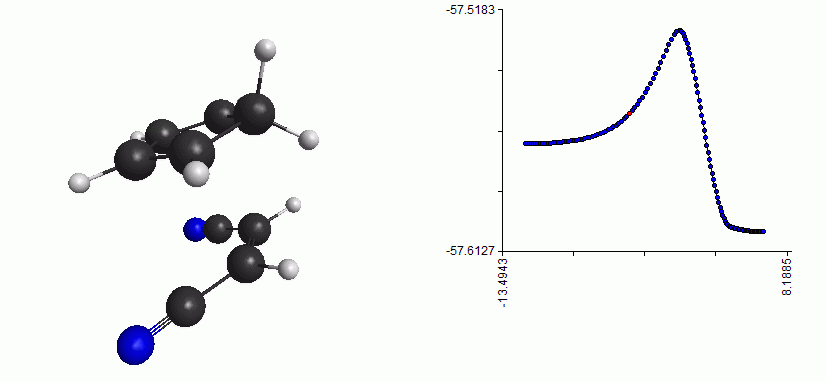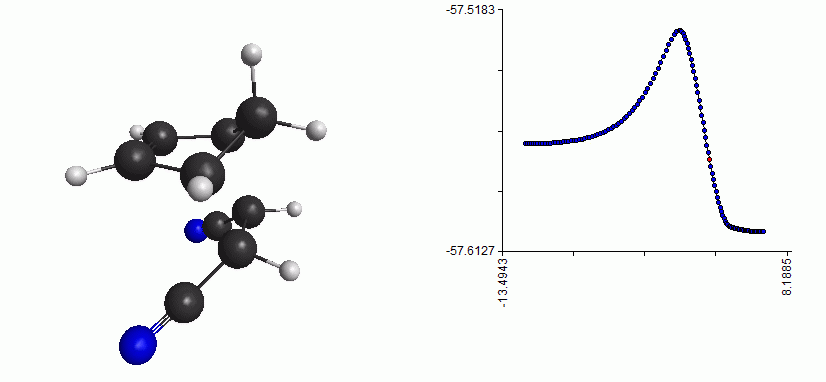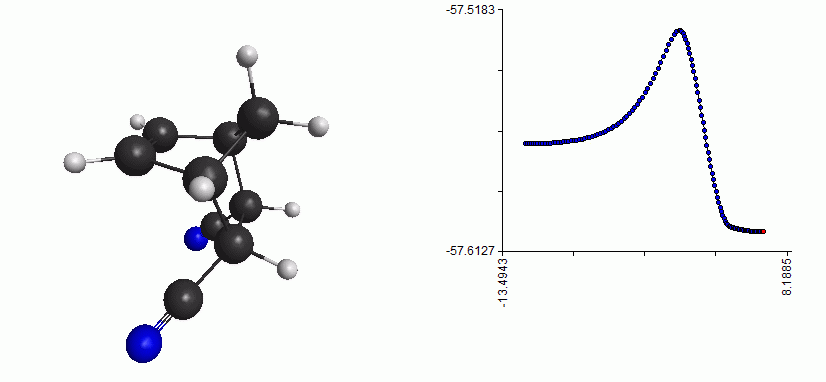
Dependence of Gibbs Energy on Temperature and Pressure. Willard Gibbs 18381903 an American physicist who first developed the concept. Gibbs free energy denoted G combines enthalpy and entropy into a single value. This will be the equilibrium point. This functionality can be formally written as.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title gibbs free energy animation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
Category
Related By Category
- 34+ Explain application of 3d animation information
- 34+ Drone gif animation info
- 20+ Fireboy and watergirl anime ideas
- 18++ Company of animals pet corrector ideas in 2021
- 13++ Animal spirit guides for money ideas in 2021
- 33+ Describe the diversity of the animal kingdom information
- 26+ Apple animals information
- 50+ Best anime movie to watch 2018 information
- 44++ Best anime figures 2019 information
- 37+ Animation of life info